In-house Research: The Administration Of Low Dose Naltrexone (LDN) For Those With Multiple Sclerosis (MS)


Harpal Clinic
Research director: Dr Harpal Bains
Research manager: Victoria Marshall
Medical team: Dr Klaudia Raczko, Dr Nathan Curran, Dr Nikita Grover
Contract manager: Ginny Laramee
Sponsors: GlycanAge, Harpal Clinic, Roseway Labs
In January 2023 we excitingly began in-house research looking at the impact that Low Dose Naltrexone (LDN) may have for those with Multiple Sclerosis (MS). Completing research was a first for us, which turned out to be an incredibly interesting and informative endeavour for our team.
LDN is an off-label treatment that encourages the body to produce its own feel-good transmitters like endorphins through a negative feedback mechanism in the body. These endorphins can help optimise the immune system and modulate it where it is hyperreactive, such as in autoimmune conditions. LDN has been used for a wide range of health concerns in patients with autoimmune diseases, cancers, HIV, MS, Systemic Lupus Erythematosus (SLE), Crohn’s and ulcerative colitis amongst others. Prior to the use of low doses of naltrexone (doses between 0.5-4.5mg), naltrexone was used in much larger doses (50-100mg) to help support people with alcohol dependency. The medical literature surrounding LDN is steadily growing, meaning that its off-label clinical application has widened. Given its extensive previous use at higher doses, you’ll often find LDN described as having a high safety profile in studies.
According to the National Institute for Health and Care Excellence (NICE): “Medicines can be prescribed if they don’t have a licence (unlicensed) or for ‘off-label’ use. Off-label means that the person prescribing the medicine wants to use it in a different way than that stated in its licence. This could mean using the medicine for a different condition or a different group of patients, or it could mean a change in the dose or that the medicine is taken in a different way.”
Another example of an off label medication people may be familiar with is Semaglutide (Wegovy). Although this medication was initially approved to help manage Type 2 diabetes, it is now being prescribed as an off-label medication to support weight loss.
We see many patients looking to use LDN at our clinic, both within functional medicine capacity as well as for longevity-associated and preventative healthcare; meaning that our team is incredibly well versed in supporting patients wishing to use LDN.
The scientific literature looking at LDN and MS is one of the most prominent areas of research for off-label use of LDN. The primary objective of the research was to determine if GlycanAge scores, individual glycans or any GlycanAge traits show any significant changes with LDN administration in a small cohort of patients with MS. Secondary to this, we were also looking to see if there is an association with the aforementioned glycan parameters and MS symptoms in the same group – measured using our carefully curated symptom severity questionnaire (SSQ).



Some of the inclusion & exclusion criteria included:
The research all took place remotely and participants were recruited through online advertising via; the MS charity MS-UK, through all research sponsor social media accounts (GlycanAge, Harpal Clinic, Roseway labs), as well as an internal email campaign. The research began with a total of 18 participants, with 10 participants remaining at the final data collection time point.
The therapeutic dose for LDN is 4.5mg per day, either sublingually or as capsules, however, anyone beginning to take LDN must initially follow a titration process first using the sublingual form. All LDN used in our research was formulated by Roseway Labs, a compounding pharmacy based in the UK.
Each patient began on a dose of 0.5mg of LDN per day, administered sublingually under the tongue. The participants were then asked to titrate up the dose every 1-2 weeks, at a pace that they felt comfortable with until they reached the therapeutic dose of 4.5mg per day. Patients will differ in how quickly they can titrate up using LDN. The participant would then remain on this dose, with the option to change the medication form to capsules for ease of administration.
We gathered GlycanAge testing and SSQ data at 3 separate time points;
We allowed participants to sign up for the research at varying time points between January-April 2023. GlycanAge at-home finger prick blood tests were sent via post, and the SSQ and personal diary were both sent via email.

Our data collection included; GlycanAge testing, our SSQ and a personal diary kept by each participant.
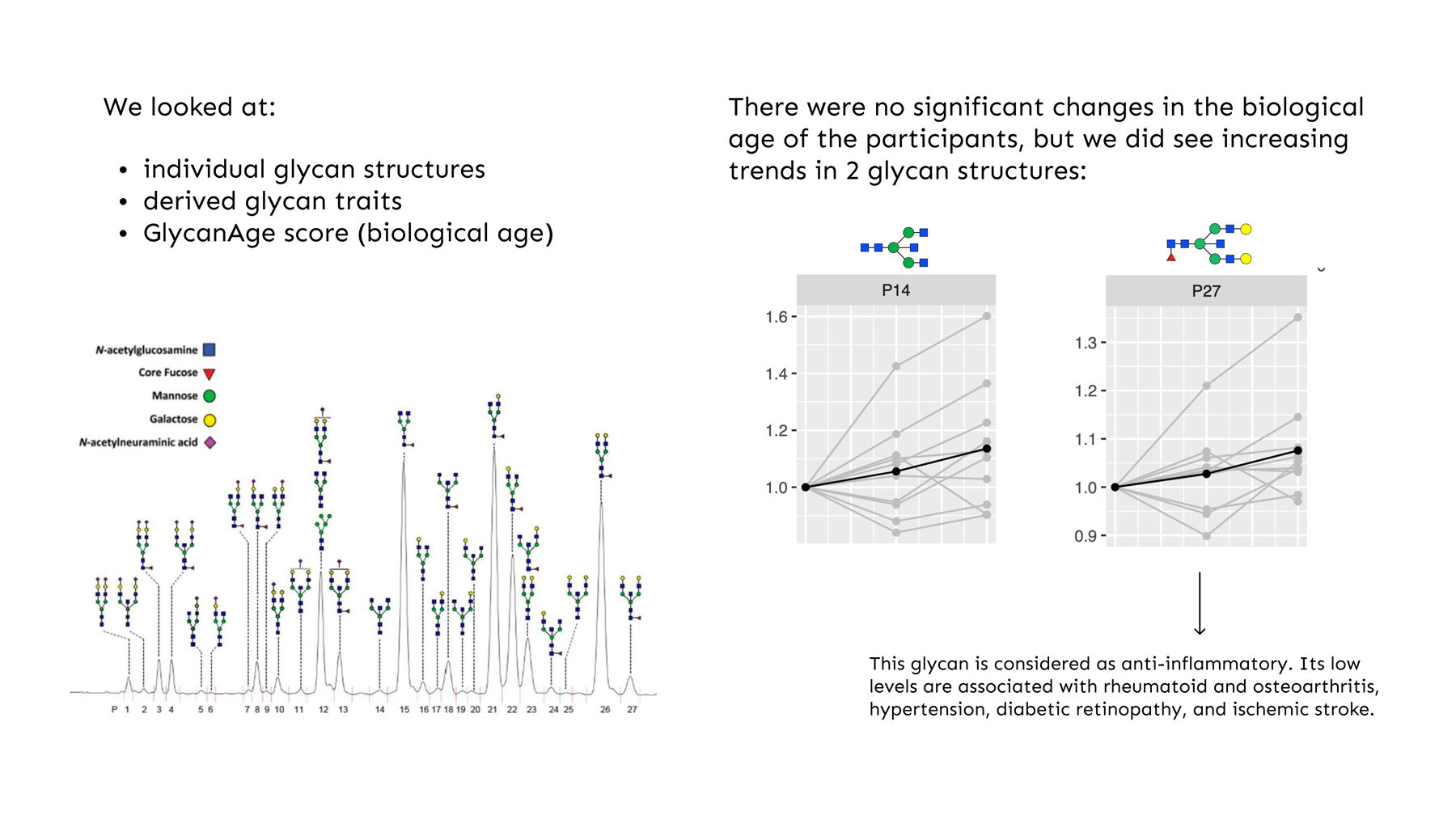
GlycanAge testing has become a prominent health optimisation test in recent years, providing an accurate biological age for individuals. It also provides an expanded report, showing a deeper analysis of a person’s individual glycan activity.
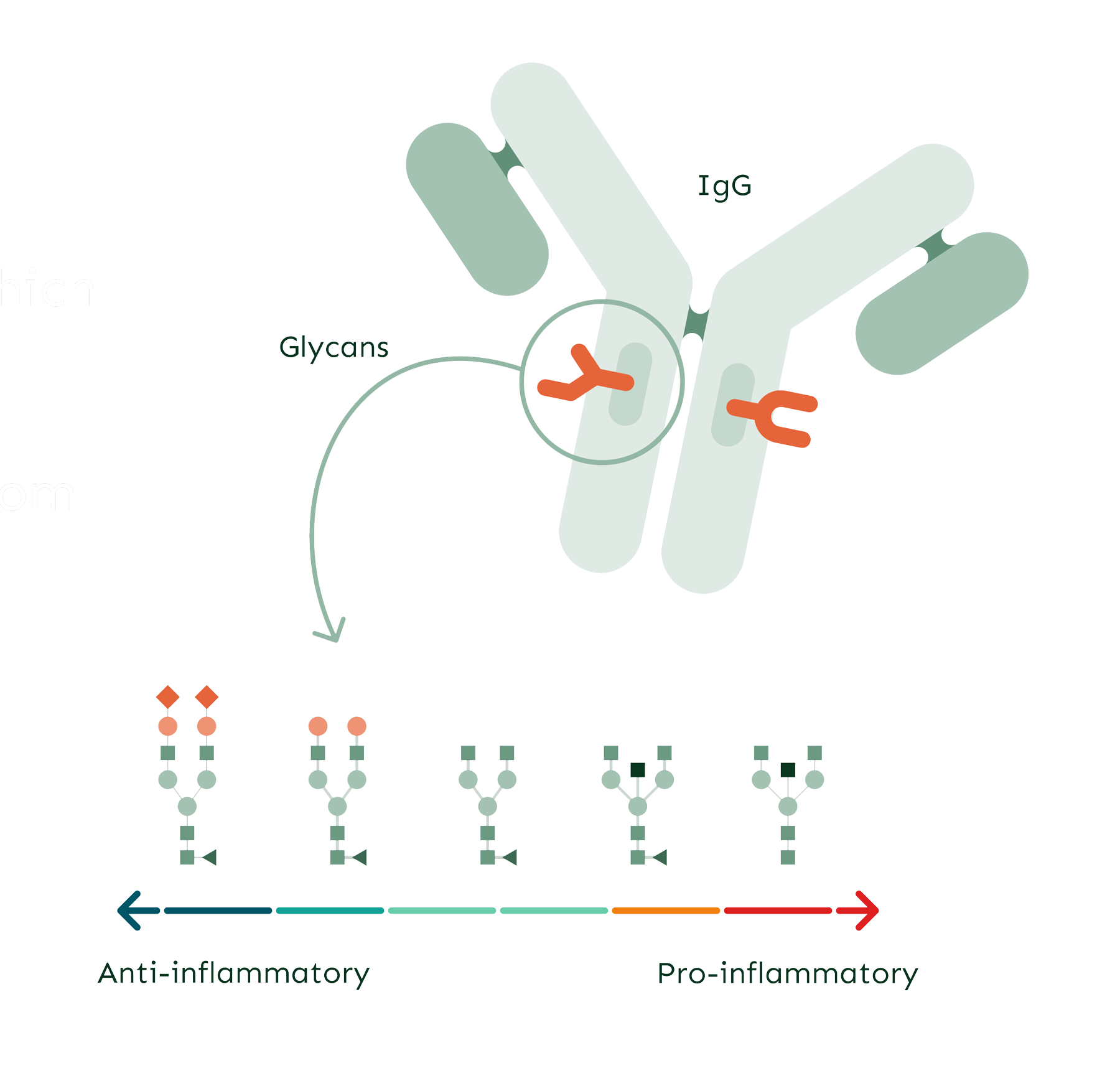
Glycans are complex carbohydrate molecules and are one of four building blocks of life (alongside proteins, lipids and nucleic acids). They are involved in the inflammatory response, including immune cells of innate and adaptive immunity. But glycans do much more than decorate proteins, as they modify the structure and function of proteins – which is why they play such an important role in various biological and physiological processes. Some of the most interesting glycans are the ones that are attached to an IgG (immunoglobulin G). IgG is the most abundant immunoglobulin and functional protein within the adaptive immune system. IgG glycans are closely associated with low-grade inflammation, making them valuable indicators of biological age and healthy ageing – especially if we wish to take a preventative approach to medicine.
GlycanAge is based on the analysis of IgG glycans which are molecular effectors of chronic inflammation.
IgG glycans undergo changes as we age, changing from anti- to pro-inflammatory. These changes are also evident in many chronic diseases, inclusing MS.
IgG glycans are responsive to lifestyle and medical interventions, making them good biomarkers for monitoring the efficacy of interventions targeted at lowering inflammation.

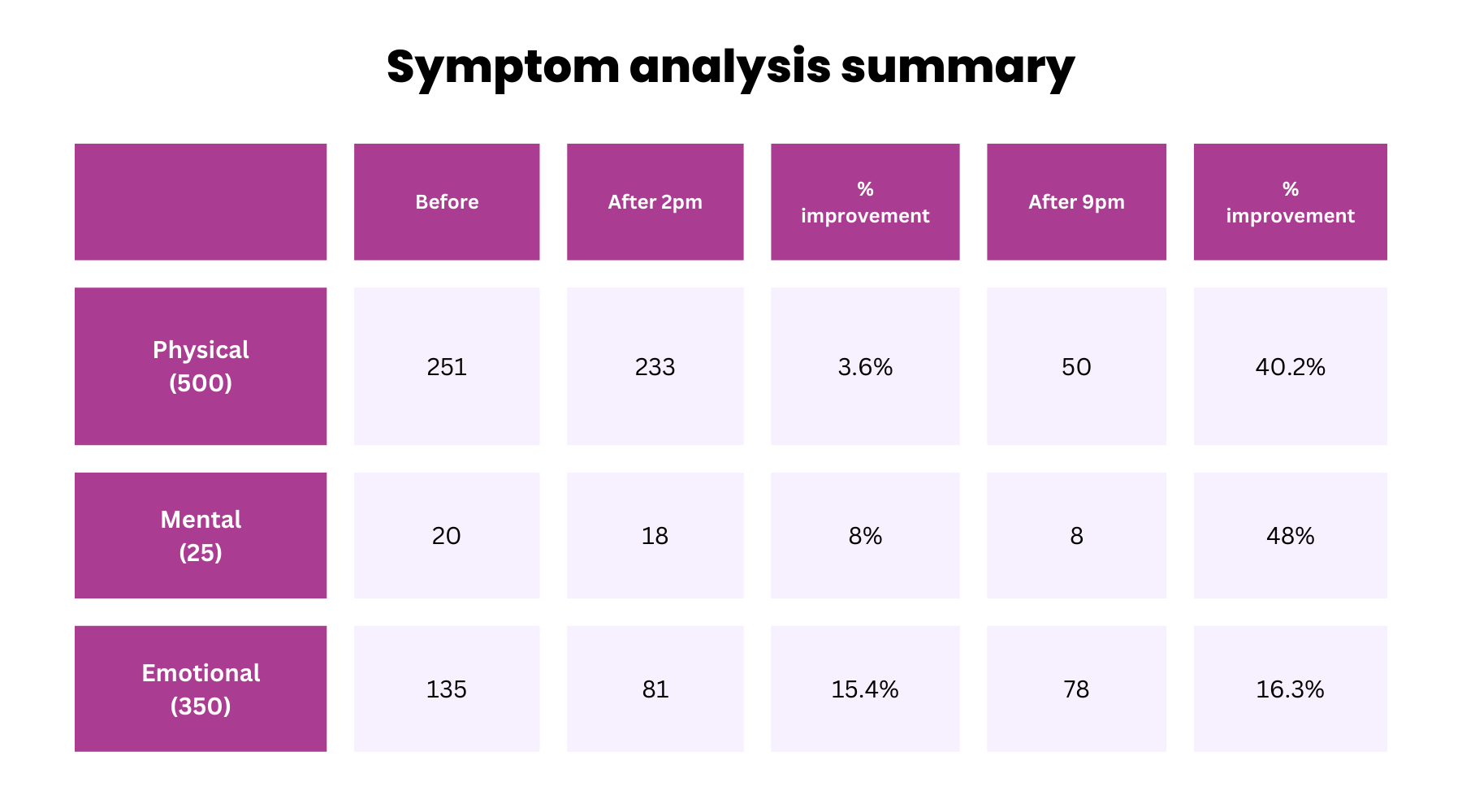
Our SSQ helped us to initially find patients that met our exclusion and inclusion criteria. The questionnaire was also repeated at each of the three timepoints by each participant. The SSQ included baseline demographics, MS-related questions (e.g., MS subtype and length of disease onset), as well as relevant lifestyle questions.
Although our SSQ and GlycanAge testing were our primary measurements, we also supplemented this by giving each participant a personal diary to complete each week. It allowed participants to make a note of any changes, and keep a log of their LDN titration.
Fig.1 Prominent glycan structures

Fig.2: SSQ analysis
We would like to say a special thank you to all of our participants, co-sponsors GlycanAge and Roseway Labs, and the whole team at Harpal Clinic for making this happen.